Zalgen Licenses Lassa Virus Diagnostic Technology from The Scripps Research Institute
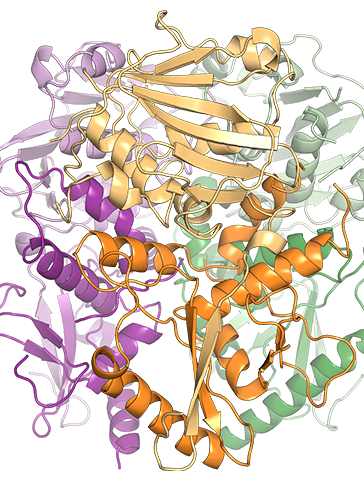
Scripps proprietary glycoprotein technology key to development of new diagnostic tests enabling the design of better, more effective vaccine programs for Lassa fever Zalgen Labs LLC (Zalgen), a biotechnology and diagnostics company focused on high-impact, neglected infectious diseases including Lassa Fever (LF), today announced that it has licensed proprietary technology developed by structural biologist Erica […]
Zalgen Licenses Lassa Virus Diagnostic Technology from The Scripps Research Institute Read More »