Executive Leadership
Zalgen Labs is a privately held Maryland Limited Liability Corporation. Established by co-founders Dr. Luis Branco and Dr. Robert Garry in 2011, Zalgen has built a team of skilled and experienced scientists focusing on basic science and product development for the improvement of humankind. At Zalgen, we treat our customers, employees and patients who benefit from our products with the highest level of respect and admiration.
Luis M. Branco, PhD
Managing Director and Co-Founder
Robert F. Garry Jr., PhD
Chief Scientific Advisor and Co-Founder
Matthew L. Boisen, PhD
Director of Diagnostics Development
Douglass T. Simpson
Senior Advisor
Vision
Zalgen integrates passion for discoveries and advancement in the biological sciences with uncompromising commitment toward fulfilling diagnostic, preventive, and therapeutic solutions for unmet and underrepresented medical needs worldwide, through excellence, nobility, proven execution, and innovation.
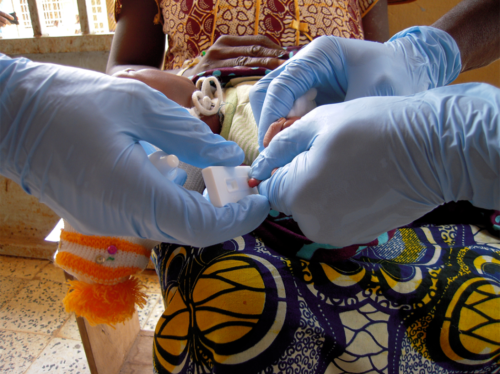
Young patient being tested for Lassa fever with ReLASV Antigen Rapid Test.
Kenema Government Hospital, Kenema, Sierra Leone. (2009) Photo courtesy L Branco
History
Zalgen Labs is a biotechnology company founded in 2011, specializing in the design and production of superior biological molecules critical for the development and commercialization of immunotherapeutics, novel vaccines, and reliable, rapid, and affordable diagnostic platforms targeting neglected and underrepresented human viral infectious diseases including Ebola virus (EBOV) and Lassa virus (LASV). Zalgen technology and products strengthen global capabilities in biosafety, biosecurity and readiness for pathogen outbreaks (most importantly viral hemorrhagic fevers) and support medical countermeasure efforts directed to pandemic diseases and major epidemic-prone diseases.
In the past few years we and our collaborative partners achieved significant performance milestones including securing over $140 million in grant funding, developed and launched the first rapid diagnostic test for Lassa fever, developed and launched the first rapid test for Ebola viral disease to secure emergency use authorization from both the US FDA and the WHO, opened state of the art biotechnology facilities in Maryland and Colorado, published 24 scientific papers in peer-reviewed journals, and established the company as a premier provider of diagnostics, research reagents, and product development solutions for high consequence neglected tropical viral diseases.
In 2020, in response to the global SARS CoV-2 pandemic, we quickly broadened our focus and began development a battery of diagnostic tests, the first of which has been submitted to the FDA for emergency use authorization request.

(L-R) Dr. Robert Garry, Dr. Jessica Grove (Hartnett), Lassa fever survivor (with permission), and Dr. Luis Branco.
Young survivor of severe Lassa fever at Kenema Government Hospital, Eastern Sierra Leone, 2010.
The patient, with family permission, helped our team assemble the most comprehensive longitudinal study
of a severe Lassa fever infection with positive outcome recorded to date.
Company Overview
At Zalgen we are dedicated to making a meaningful difference in developing and deploying medical countermeasures against neglected tropical diseases. Since our inception, we and our academic, government, biotechnology industry and healthcare provider partners have made significant strides against Ebola, Lassa and other highly fatal hemorrhagic viral diseases faced in resource limited areas of the world desperate for resources to enhance scientific research and improve patient care.
Our teams have traveled frequently to Sierra Leone, Nigeria and other West African countries, often during disease outbreaks. Working with scientists and healthcare workers, Zalgen has made measurable advances in the science of viral hemorrhagic diseases to improve the lives of these severe at-risk populations, impacted by these diseases and associated mortality rates approaching or exceeding 70%, and even higher in pregnant women and children.
Our goal is to develop and make available the best, most widely trusted and effective diagnostics, vaccines and immunotherapeutics to make a meaningful difference against these endemic and epidemic diseases. With active and broadly disseminated vaccine programs, field deployable rapid diagnostic tests, and availability of affordable and safe immunotherapeutics, celebrations with young survivors will be the norm rather than just the fortunate few who survive these diseases.
Science Leadership
Zalgen is on the cutting edge of viral hemorrhagic fever products discovery and development.
Our scientific team, advisors and collaborators have the experience and know-how to navigate through this difficult environment in a very efficient manner that will bring to market numerous innovative and useful products critical to combat these fevers and related neglected tropical diseases.
Led by Dr. Robert F. Garry, Zalgen programs cover three important business areas — vaccines, immunotherapeutics and diagnostics — all utilizing proprietary recombinant technology and platform experience resulting in solutions addressing critical medical needs.

Robert F. Garry, Ph.D.
Chief Scientific Advisor
B.S. (1974) Indiana State University, Life Sciences
Ph.D. (1978) University of Texas at Austin, Microbiology
Postdoctoral Research (1978-82) University of Texas at Austin
Dr. Garry is the President of the Viral Hemorrhagic Fever Consortium (VHFC), a non-profit consortium of scientists developing countermeasures including diagnostics, immunotherapeutics and vaccines, against Lassa virus, Ebola and Marburg viruses and other high consequence pathogens. Dr. Garry serves in the Tulane University administration as Assistant Dean for Graduate Studies in Biomedical Sciences and is the founding Editor-in-Chief of Virology Journal.
Raju Lathigra, Ph.D.
Program Director: Molecular Biology
B.Sc. (1975) Sardar Patel University, Microbiology
M.Sc. (1978) Maharaja Sayajirao University, Microbiology
Ph.D. (1983) Queen Mary, University of London, Microbiology and Molecular Biology
Dr. Lathigra is involved in developing bispecific antibodies against LASV and EBOV as second and potentially 3rd generation therapeutics based on Arevirumab and EBOLA antibodies. At MedImmune he was also involved in engineering second generation derivatives of Synagis. Dr. Lathigra has over 30 years of experience in bacteriology and has worked on mycobacteria, Lyme disease, and organisms that cause otitis media. He made key contributions for the determination of the whole genome DNA sequence of Lyme disease causing bacteria which has led to expedited evaluation of potential vaccine candidates against Lyme disease. In his work with the US Army for 12 years, Dr. Lathigra worked with high impact BSL-3 category infectious disease agents Bacillus anthracis, Francisella tularensis and Yersinia pestis.
Corporate Responsibility
The Zalgen organization is committed to highly ethical and responsible behavior in all that we do as individuals and as a team. We have in place policies and procedures resulting in consistent quality of our work product and ensuring all members of our staff are treated with the high level of respect they deserve. New employees are selected, trained and expected to commit to this same high level of ethical behavior.
Zalgen Policies and Procedures include: Adherence to the Research Investigators’ Financial Conflict of Interest (“RIFCOI”) regulations. This includes “Responsibility of Applicants for Promoting Objectivity in Research for which Public Health Service Funding is Sought” (42 CFR Part 50, Subpart F), and “Responsible Prospective Contractors” (45 CFR Part 94).
Careers
Zalgen Labs is continuously seeking talented, highly committed people to join our organization. If you are interested in joining a fast-paced biotechnology business, we want to hear from you.
Zalgen Locations
Frederick, Maryland — Headquarters & Biotechnology Center
Aurora, Colorado — Diagnostics Technology Center
Positions currently available.
Zalgen Labs has been and will continue to be an equal opportunity employer. The Company will continue to take steps to assure that recruitment, hiring, assignment, promotion, compensation and all other personnel decisions are made and administered without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, ancestry, age (forty and above unless defined more broadly under applicable state law), mental and/or physical disability, genetic information, veteran status, any military service or application for military service, marital status, creed (belief system), citizenship status or membership in any other category protected under state, federal or local law.




